WINMED’s Women Healthcare business unit is committed to several other aspects of female healthcare. Our range of products which is constantly expanding, now includes Maternal-Fetal medicine. This comprises of Down’s syndrome and genetic screening test, pregnancy monitoring and cervical dilators.
Benefits of the ThinPrep Pap Test include; increased screening accuracy and superior patient comfort; multiple tests can be performed using one sample specimen, which limits the need for further samples to be collected. The ThinPrep Pap Test Provides screening for human papillomavirus (HPV) making it an invaluable step in establishing all-round awareness of patient’s state of health.
For further information
PRODUCT SPECIFICATION

To identify high-risk HPV infections by targeting E6/E7 mRNA. Studies show that targeting E6/E7 mRNA identifies the presence and activity of a high-risk HPV infection. The APT HPV ASSEY represents the next generation in cervical screening – allowing clinicians to deliver maximum benefit to patients, while minimizing potential harm.
The APT HPV ASSEY identifies patients at greatest risk of developing cervical cancer, delivering the same excellent sensitivity to minimize false negative results. The APT HPV ASSEY demonstrates the same excellent sensitivity as DNA-based HPV tests innumerous clinical studies involving over 50,000 women worldwide.
For further information
PRODUCT SPECIFICATIONThe Centers for Disease Control and Prevention (CDC) estimates that only 38% of sexually active young women are screened annually for chlamydia in the United States and that up to 75% of CT infected women are asymptomatic contributing to as many as 30% of CT infections progressing to pelvic inflammatory disease (PID) which has been shown to cause permanent damage to a woman’s reproductive system including infertility, chronic pelvic pain and ectopic pregnancy.
For further information
PRODUCT SPECIFICATION
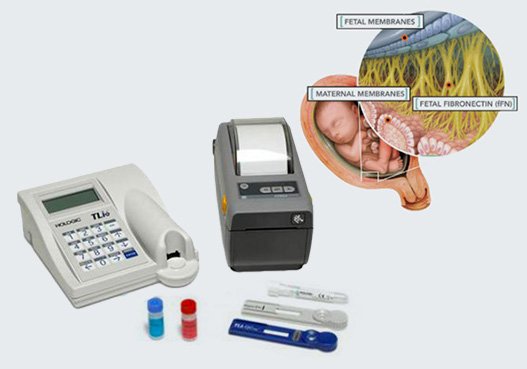
The fFN test presents a more reliable method of determining the likelihood of preterm birth. Administered similarly to a Pap Test, the fFN test is a safe and non-invasive method of measuring fFN levels in vaginal secretions. Usually, fFN can only be detected at the beginning of pregnancy and towards full term. Detectable levels of fFN from weeks 22 to 35 of pregnancy are a strong indicator that the patient is at high risk of delivering their baby preterm.
The fFN is valuable for its role in identifying patients who are not at risk for preterm birth. Mothers whose symptoms would otherwise be addressed with medication or lying-in can avoid disruption to their daily lives.
For further information
PRODUCT SPECIFICATIONบริษัทเป็นตัวแทนจำหน่ายกล้องส่องปากมดลูก (Colposcope) เพื่อตอบโจทย์สูตินรีแพทย์แบบครบวงจรโดยต่อยอดจากการตรวจมะเร็งปากมดลูก โดยนำเข้าเครื่องคอลโปสโคประบบใหญ่จากบริษัท ไซเลอร์ ประเทศสหรัฐอเมริกา ซึ่งเป็นผู้นำเทคโนโลยีขั้นสูงกล้องส่องขยายในอุตสาหกรรมทางการแพทย์ ทันตกรรม และจักษุแพทย์ อีกทั้งยังนำเข้าเครื่องคอลโปสโคประบบพกพาที่ง่ายต่อใช้งานแต่ยังคงคุณภาพสูง จากบริษัท โมบาย โอดีที จากประเทศอิสราเอล อีกด้วย

